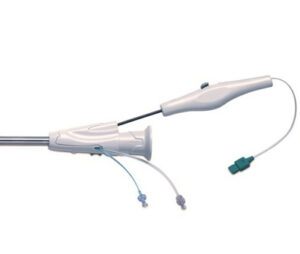
FDA issues Class I recall for medical devices due to silicone separation
Washington, DC – The FDA has issued a recall for certain VasoView HemoPro Endoscopic Vessel Harvesting (EVH) Systems, adding these devices to the medical device shortage list on November 15, 2024. This recall, classified as Class I, the most serious type, involves removing the affected devices from use or sale. The devices in question are detailed in a Letter to Health Care Providers, which contains the most current information.
The recall affects VasoView HemoPro 1 (VH-3000-W) and 1.5 (VH-3500) models, identified by their unique device identifiers. Getinge, the manufacturer, sent an Urgent Medical Device Removal letter to all affected customers on September 20, 2024. The letter advises customers to examine their inventory, remove the affected devices, and contact Getinge for return authorization and shipping instructions for unused or unexpired products.
The recall was initiated due to the risk of silicone detaching from the harvesting tool during use, potentially causing silicone debris to enter the patient. This defect can render the device non-functional and may lead to serious health consequences, including blood vessel blockage, infection, and even death. Although there have been 17 reported injuries, no deaths have been reported.
Customers are urged to complete and return the Medical Device Removal Response Form included in the recall letter, regardless of whether they have affected products. They should also forward this information to all potential users within their facilities. For further assistance, customers can contact Maquet Cardiovascular/Getinge Customer Support at 1-888-880-2874.