Researchers develop new styrene production method improving stability, dehydration activity
Tsinghua, China – A team of researchers led by Hongyang Liu of the Chinese Academy of Sciences Institute of Metal Research, has developed a method of ethylbenzene dehydrogenation under oxygen-free conditions with fully exposed platinum (Pt) cluster catalysts that result in the positive traits of high activity, selectivity and stability, as well as lower energy and financial costs. The results will be published July 10 in Nano Research.
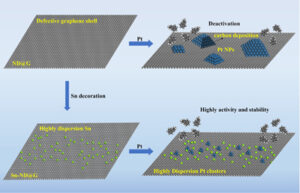
“We have prepared fully exposed Pt cluster catalysts by exploiting the carbon defects on the surface of the graphene support and the physical segregation of atomically dispersed tin (Sn),” said Liu, who is also appointed at the University of Science and Technology of China. “The fully exposed Pt clusters can promote the desorption of the target product styrene, making it exhibit higher dehydrogenation activity and stability than Pt nanoparticles catalysts.”
In contrast, a common previous method of ethylbenzene dehydrogenation took place over iron oxide-based catalysts, required high temperatures that result in carbon deposition and required excess superheated steam. To overcome this, researchers have employed single-atom catalysts (SAC) and fully exposed cluster catalysts (FECCS).
“SACs and FECCs deliver a wide range of atomic dispersion and full utilization efficiency of the metals, which can provide enhanced activity and have received a great deal of interest,” Liu said. “Especially, the active sites of FECCs generally contain diverse combinations of multiple metal atoms and are suitable for catalyzing reactants that need ensemble metal sites.”
However, SACs and FECCs, have their own limitations, including imprecise control of the structure uniformity of FECCs and aggregation of metal atoms into metal clusters or nanoparticles caused by their high surface energy and thermodynamic instability when exposed to high temperatures.
While other researchers have aimed to design FECCs with high activity and high stability suitable for high-temperature reactions such as ethylbenzene dehydrogenation, as this team of researchers did, previous studies used non-precious metal oxides or carbon materials for catalysts, which require high energy and water consumption and result in low activity. The energy consumption can be addressed by oxidization of the process, but that leads to low selectivity and hazards with flammable mixtures.